Developing a Research Base for Intravenous Peripheral Cannula Resites Essay
Developing a Research Base for Intravenous Peripheral Cannula Resites Essay
Two hypotheses were tested in this paper. There are two groups that were compared in the paper. The control group has participants who follow the three-day cannula re-site protocol currently employed in most hospitals. The intervention group would only have cannula re-sites if their medical condition demands it. One of the hypotheses of this paper states that the control group would have more unplanned IV cannula re-site than the intervention group. Secondly, it also states that the cost of total cannula re-site of the control group would be higher than in the intervention group.
A randomized controlled trial was employed for this study. This was very much appropriate for this type of study. According to Pildal, et al (2005), Randomized Controlled Trial or RCT is best for medical or health-related studies. It ensures efficiency and accuracy as long as the number of subjects is enough to represent the population. In addition, confounding may be prevented by using this type of research method. Developing a Research Base for Intravenous Peripheral Cannula Resites Essay.
The control group followed the protocol of transferring the peripheral venous catheter to a different site every 3 days or as clinically indicated. On the other hand, the intervention group only transferred the peripheral catheter when clinically indicated.
The independent variable for this research is the relocation of the peripheral venous catheter. Treatment for this variable is transfer every 3 days and transfer only as clinically necessary. This variable is constant.
The dependent variable, on the contrary, is the cost of cannulation and the number of unplanned cannula re-sites. This variable is highly dependent on the two treatments described above. The total costs of cannula re-sites and the actual number of unplanned transfers would vary depending on the treatment of the independent variable.
Background: There is currently no high grade evidence on which to base decisions about the frequency of intravenous cannula re-sites.
Objective: To assess the safety of changing peripheral venous cannulas when clinically indicated.
Design: Randomised controlled trial.
Setting: A tertiary referral hospital in Brisbane, Australia.
Participants: Two hundred and six hospitalised patients from surgical, medical and orthopaedic wards.
Interventions: Peripheral intravenous cannulas were re-sited only when complications occurred (intervention group) or every 3 days (control group).
Main outcome measures: The primary endpoint was any unplanned cannula removal, the secondary outcome was cost. Developing a Research Base for Intravenous Peripheral Cannula Resites Essay.
ORDER A PLAGIARISM-FREE PAPER NOW
Results: Forty six patients had unplanned removals in the intervention group compared with 41 in the control group [relative risk 1.12, 95% confidence interval 0.81-1.55 (p=0.286)], a non-significant difference. Total duration of peripheral cannulation was similar in both groups (mean 123.3h in the intervention group and 125.9h in the control group: P=0.82) but significantly more re-sites occurred in the control group (167 in intervention group, 202 in the control group: p=0.022). Cost of cannula replacements in the intervention group was AUD$3,183.62 and in the control group AUD$3,837.56 (p=0.006).
Conclusion: Re-siting peripheral venous cannulas when clinically indicated compared with changing them routinely every 3 days does not lead to more complications and reduces costs.
Peripheral intravenous device (IVD) complications were traditionally thought to be reduced by limiting dwell time. Current recommendations are to resite IVDs by 96 hours with the exception of children and patients with poor veins. Recent evidence suggests routine resite is unnecessary, at least if devices are inserted by a specialised IV team. The aim of this study was to compare the impact of peripheral IVD ‘routine resite’ with ‘removal on clinical indication’ on IVD complications in a general hospital without an IV team.
Methods
A randomised, controlled trial was conducted in a regional teaching hospital. After ethics approval, 362 patients (603 IVDs) were randomised to have IVDs replaced on clinical indication (185 patients) or routine change every 3 days (177 patients). IVDs were inserted and managed by the general hospital medical and nursing staff; there was no IV team. Developing a Research Base for Intravenous Peripheral Cannula Resites Essay.The primary endpoint was a composite of IVD complications: phlebitis, infiltration, occlusion, accidental removal, local infection, and device-related bloodstream infection.
Results
IVD complication rates were 68 per 1,000 IVD days (clinically indicated) and 66 per 1,000 IVD days (routine replacement) (P = 0.86; HR 1.03; 95% CI, 0.74-1.43). Time to first complication per patient did not differ between groups (KM with log rank, P = 0.53). There were no local infections or IVD-related bloodstream infections in either group. IV therapy duration did not differ between groups (P = 0.22), but more (P = 0.004) IVDs were placed per patient in the routine replacement (mean, 1.8) than the clinical indication group (mean, 1.5), with significantly higher hospital costs per patient (P < 0.001).
Conclusions
Resite on clinical indication would allow one in two patients to have a single cannula per course of IV treatment, as opposed to one in five patients managed with routine resite; overall complication rates appear similar. Clinically indicated resite would achieve savings in equipment, staff time and patient discomfort. There is growing evidence to support the extended use of peripheral IVDs with removal only on clinical indication.
Registration number
Australian New Zealand Clinical Trials Registry (ANZCTR) Number ACTRN12608000421336.
Background
Peripheral intravenous device (IVD) insertion is the most commonly performed invasive procedure in hospitalised patients, with an estimated 150 million peripheral intravenous devices placed each year in North America alone [1]. IVDs are vital for delivery of hydration, medicines and nutrition but are not without complications. Serious adverse outcomes are fortunately rare, with IVD-related bloodstream infection reported in a recent meta-analysis of 110 studies to occur in 0.1% of devices and 0.5 per 1,000 device days [2]. IVD treatment is more frequently interrupted by phlebitis, an irritation of the vein characterised by pain, tenderness on palpation, erythema, warmth, swelling, induration or palpable cord (thrombosis) of the cannulated vein; diagnostic algorithms usually require two or more of these conditions [3–5]. Phlebitis is in almost all cases a biochemical reaction to the mechanical irritation by the presence of the IVD and associated infusate [3], although phlebitis symptoms such as erythema may be misperceived as indicative of an infection. In fact, there is not a high correlation between phlebitis and device infection, and the Centers for Disease Control (CDC) states that infection is rarely associated with peripheral, as opposed to central, venous devices [3, 6, 7]. Fluid infiltration or ’tissuing’ of devices is another common IVD complication which may also reflect the inflammatory (phlebitic) response of the vein, rather than simple misplacement of the device tip [8]. Developing a Research Base for Intravenous Peripheral Cannula Resites Essay.
Early cohort studies noted an association between increased device time in situ and phlebitis [9, 10]. This association was responded to with policies for routine device removal. Recommended timelines for routine resite have been extended over the past three decades from 24, to 48, then to 72 hours. Currently, 72- to 96-hour resite is recommended to reduce phlebitis by the CDC’s 2002 Guidelines for the Prevention of Intravascular Device Infection, with the exemption that this is not required in children or those with poor veins [7]. Such policies cause increased workload in hospitals, where the task of removing and replacing well-functioning IVDs generally falls to busy nursing and junior medical staff. In addition, few patients welcome the prospect of additional venipuncture.
Despite the general clinical acceptance of routine IVD replacement as a phlebitis and infection prevention measure, it has not been supported by recent data. It may be that the risk of complications during the entire IVD treatment episode is similar, regardless of whether multiple short-dwell or fewer longer-dwell IVDs are used over this time. Three small (n = 47-60) randomised, controlled trials (RCTs) suggested routine replacement at 12, 24 or 48 hours may reduce phlebitis compared to resite on clinical indication, although a systematic review for the Swedish Council on Technology Assessment in Healthcare assessed these as low- to medium-quality studies providing ‘limited scientific evidence’ [11–14]. More recently, two well-conducted RCTs found no evidence of effect when comparing IVD replacement every 3 days with replacement only on clinical indication for medical and surgical inpatients [15, 16]. The largest of these studies reported findings from 755 general medical and surgical patients with 1,428 IVDs and found a 5% difference in combined phlebitis and infiltration rates per patient (38% clinically indicated resite, 33% routine resite), suggesting a potential small clinical benefit of 3-day resite [15]. Developing a Research Base for Intravenous Peripheral Cannula Resites Essay.However, this difference was not statistically significant (RR 1.15; 95% CI, 0.95-1.40) and disappeared when overall cannulation time was considered (59.8/1,000 IVD days clinically indicated resite, 60.9/1,000 IVD days routine resite; RR 0.98; 95% CI 0.78-1.24) [15]. In addition, no clinically important or statistically significant differences were observed in the secondary endpoints of phlebitis, infiltration, occlusion, local infection or suspected bloodstream infection rates between study groups [15]. Another recent RCT in the ‘hospital in the home’ community setting also found no important clinical or statistically significant difference in phlebitis, occlusion or bloodstream infection rates in 316 patients when resite every 3 days was compared with clinically indicated resite [17]. A 2010 Cochrane Collaboration review concluded there was ‘no conclusive evidence of benefit’ of routine IVD resite and suggested organisations could consider adopting a resite on clinical indication policy [18]. There is growing evidence that routine IVD replacement may be ineffective, although caution has been urged in light of the large number (74% in both groups in the largest study to date) of reported devices inserted by a specialised IV team, a factor known to reduce complications [19].
Device insertion (and reinsertion) is unpleasant for patients, requires skilled and available clinical staff, and has associated costs for the health sector. If replacement only on clinical indication is safe and effective, this would have important benefits for patients and the health system. We report a RCT of 3-day routine IVD resite versus clinically indicated replacement in a medical-surgical hospital where IVDs were inserted by the general medical and nursing staff; the insitution did not have a specialised IV service. Developing a Research Base for Intravenous Peripheral Cannula Resites Essay.
Methods
Aim
The aim of the study was to compare the impact of 3-day routine resite, with clinically indicated resite, on peripheral IVD complications.
Design
Open (nonblinded), parallel group RCT.
Ethics
The study was approved by the Tasmanian State Human Research Ethics Committee. Written informed consent was obtained prospectively from all participants.
Setting and sample
The study was undertaken at a large regional teaching hospital in Australia which functions as the tertiary referral centre for the northern half of the State of Tasmania. The hospital has more than 32,000 separations per annum, with a spectrum of medical and surgical specialties. Eligible patients were at least 18 years of age and scheduled or expected to have a peripheral IVD indwelling for at least 4 days, and they gave written informed consent. Exclusion criteria were immunosuppression, current bloodstream infection or an IVD already in situ for >48 hours. Developing a Research Base for Intravenous Peripheral Cannula Resites Essay. IVDs were inserted and cared for by the general nursing and medical staff; there was no special IV team or service.
Sample Size
Sample size calculations were performed using PASS 2008 (Version 8.0.8; Kaysville, UT) to detect a change in rates by 30% (from 36% to 25%, two-tailed α = 0.05, 90% power) on the basis of the complication rates of routinely resited IVs in a previous study [16]. Although this indicated that n = 378 per group (total 756) were required, the study was ceased early (total n = 606 IVs) because all investigators left the employment of the institution. Consequently, study power was reduced, but remained over 80% (required minimum n = 282 per group).
Recruitment
All adult patients admitted to the inpatient acute medical and surgical wards of the study hospital were screened by a full-time research nurse. This excluded paediatric, day-surgery, mental health, obstetric, critical care and dialysis units.
Study procedures
Patients were randomly assigned (computer generated) in a 1:1 allocation ratio to either the ‘routine replacement’ (control) or ‘clinically indicated replacement’ (intervention) group. Assignment was concealed until randomisation by use of a telephone service. A tag was placed on the insertion site indicating the study group. All devices for the patient were managed as per randomised group. The intervention group did not have their IVD resited unless clinically indicated. This decision was made by the treating clinician (not the investigators), who ordered IVD resite if the device failed or phlebitis occurred and ongoing IV treatment was required. The control group had a new device relocated to a different site by the general medical or nursing staff every 3 days. Control devices could also be removed at any stage by the clinical staff if they were not required or if complications occurred. Developing a Research Base for Intravenous Peripheral Cannula Resites Essay. Clinical nursing and medical staff undertook insertion and follow-up care of all IVDs as per the CDC Guidelines [7].
Laboratory staff undertaking microbiological culture assessments were blinded to the study group. Due to the nature of the intervention, patients, research, and clinical staff were unable to be blinded. However, the investigators had no involvement in assessing or documenting complications.
IVDs were assessed by the clinical nursing staff on each nursing shift for complications as part of standard clinical practice in the hospital. Times and dates of device insertion and removal were recorded along with the reason for device removal and any protocol deviations. A full-time research nurse collected data from the hospital chart and sought clarification from patients and clinical staff if necessary. Microbiological investigations (device tip, blood cultures and site cultures) were performed by the clinical staff on clinical suspicion of infection by the treating clinician. Demographic and clinical data were collected on age, sex, diagnosis at hospital admission, phlebitis risk based on Tagar et al.’s classification (low/medium/high risk) [20], past history of phlebitis, any comorbidities requiring active medical treatment (e.g., type 2 diabetes or congestive heart failure), haemoglobin, concurrent infection at other sites, antibiotic therapy, type of surgery, type of infusate and any additives (and their level of irritability), vein and skin quality assessment, size of device, insertion site, health professional inserting the device, and setting for insertion, presence of other vascular devices, wound drains and urinary catheters. Vein quality was assessed as good (vein easy to visualise and easy to palpate with tourniquet on), fair (not easily visible but can palpate with tourniquet), or poor (veins small, scarred or difficult to palpate with tourniquet; may require heat pack to aid vasodilation). Skin quality was assessed as good (healthy, well hydrated, elastic), fair (mildly dehydrated, reduced elasticity), or poor (papery, dehydrated, or reduced elasticity). Developing a Research Base for Intravenous Peripheral Cannula Resites Essay.
Analytic Approach
The primary outcome was a composite measure of any complication causing unplanned cannula removal prior to completion of IV treatment. The composite included phlebitis, infiltration, occlusion, accidental removal, local infection, and IV device-related bloodstream infection (IVD-BSI). These were also analysed individually as secondary endpoints. A composite measure was chosen due to the low rates of these conditions individually and to the assumption that they are comparable measures of ‘infusion failure’; that is, the device can no longer be used to deliver treatment. This approach has been used in previous studies on the topic [15–17]. Phlebitis was defined as two or more of pain, erythema, purulence, streak formation, or a palpable venous cord [3]. Local infection IVD-BSI (bacteremia/fungemia with at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infection, and no apparent source for the bloodstream infection (BSI) except the device with or without positive tip or entry site swab culture) were defined using CDC criteria [7]. Other secondary outcomes were time in situ (hours of catheterisation from insertion to removal, both per patient and per device) [7]; IVDs per patient (number of peripheral devices inserted to complete the course of treatment) [7]; costs (calculations based on 20 minutes nursing or medical insertion time at relevant rates [15], plus the cost of the required equipment (cannula, insertion pack including dressing and solution, gloves, saline, syringe, extension tubing and starter pack for all plus fluid bag, tubing and secondary tubing for medication administration for those patients requiring this) for insertions, nursing time and equipment to routinely remove IVDs that were otherwise functional, and the costs of treating any complications that occurred (e.g., IVD-BSI). Developing a Research Base for Intravenous Peripheral Cannula Resites Essay. Cost calculations were undertaken from the viewpoint of the hospital using negotiated wage costs and purchasing agreements for government hospitals in the State of Tasmania. Costs would be similar for other Australian hospitals.
All randomised patients were analysed by intention to treat. Each patient was randomised once and could have multiple IVDs, with subsequent IVD resites managed as per the randomised group. Relative incidence complication rates per 1,000 IVD days and 95% confidence intervals were calculated to summarise the impact of clinically indicated replacement relative to 3-day replacement. Kaplan-Meier survival curves were drawn to compare time to first IVD complication between patients in the two study groups. To assess for any potential impact of protocol violations, a per protocol analysis was also undertaken. All clinical and demographic variables were subjected to univariate testing against the primary endpoint to guide selection of possible covariates for the multivariable model. Cox proportional hazards regression modelling was used to examine the impact of age, gender, oncology status, number of comorbidities (nil, one, two, or more than two), IV gauge, site, vein quality, skin quality, oral antibiotics, IV antibiotics, wound drain, inserter occupation, initial versus subsequent IVDs, phlebitis in a preceeding IVD, haemoglobin level, parenteral nutrition, continuous versus intermittent infusion, patient risk category and study group on the outcome of time to complication events using an additive model [3, 5, 7, 20–25]. In addition, to adjust for any inherent correlations or codependencies in the failure times of IVDs (i.e., same patient multiple failure-time data) within the Cox model, we also used the Prentice-Williams-Peterson conditional risk-set method [26]. The Mann-Whitney test was used to compare various secondary outcomes between study groups. Cost differences were calculated using arithmetic means and the t-test [27]. P values <0.05 were considered significant. All statistical data were entered and analysed using SPSS (Version 15.0; Chicago, IL) and Stata (Version 8.2; College Station, TX). Developing a Research Base for Intravenous Peripheral Cannula Resites Essay.
Results
Sample
Over a 10-month period, 1,954 patients were screened for eligibility. Of these, 788 were eligible, with 362 (46%) recruited into the study. The most frequent exclusion criterion was altered mental state that precluded consideration of consent as assessed by the research nurse. Altered mental state was generally related to older medical patients and the immediate postoperative phase for surgical patients. Reasons for exclusion are shown in Figure 1. The 362 patients were randomised into either the routine change group (n = 177 participants, 323 devices) or the clinically indicated replacement group (n = 185 participants, 280 devices). In total 50,173 IVD hours were studied (routine change group 23,288 hours, clinically indicated group 26,885 hours). More patients in the routine change group had an active infection (53% vs. 44%) and were receiving IV antibiotics (73% vs. 64%). However, as shown in Tables 1and 2, the two groups were generally comparable at baseline for patient- and cannula-related factors.
ORDER A PLAGIARISM-FREE PAPER NOW
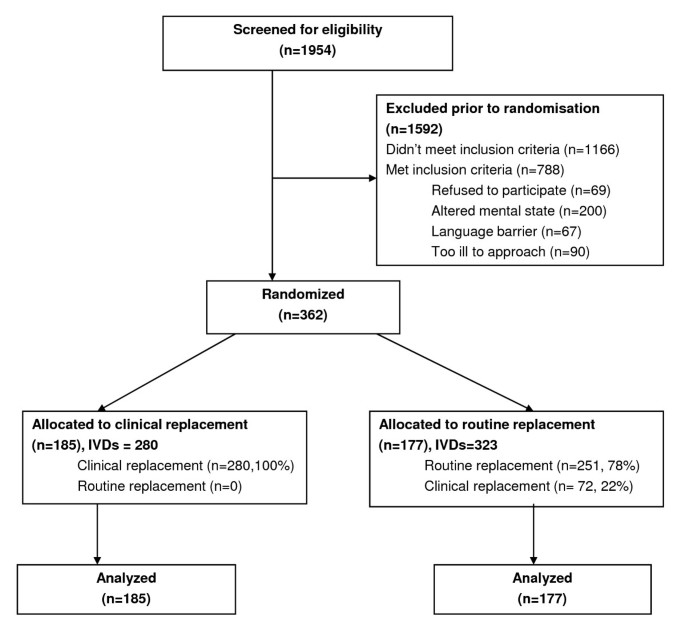
Participant flowchart.
Effect of intervention on primary outcome
Outcome data were available for all patients. Table 3 shows the rates of primary and secondary outcomes. Differences in complication rates between groups were not significantly different (routine replacement 66.0 per 1,000 IVD days; clinical replacement 67.8 per 1,000 IVD days; HR 1.03; 95% CI, 0.74-1.43; P = 0.86). As shown in Figure 2, the time to first complication per patient was also not significantly different between groups (Kaplan Meier [KM] with log rank P = 0.53). On crude rate per IVD, the catheters replaced on clinical indication had higher complication rates (110/280 or 39% vs. 91/323 or 28%; P = 0.004). However, total complication rates per patient (to deliver the course of IV therapy) were not significantly different (P = 0.39) between clinically indicated (76/185, 41%) and routine resite patients (64/177, 36%). Developing a Research Base for Intravenous Peripheral Cannula Resites Essay.
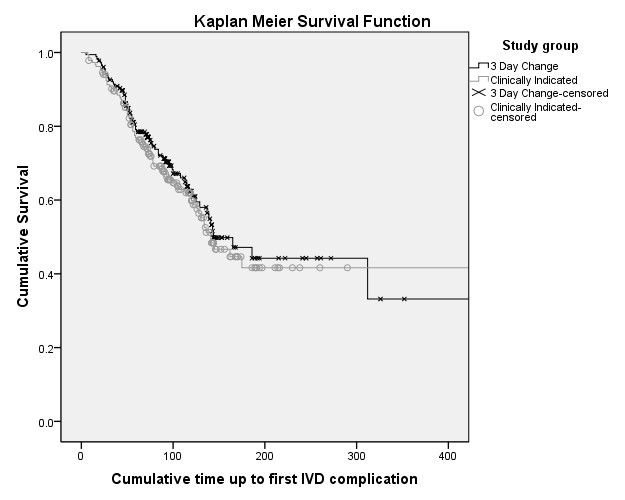
Kaplan-Meier survival curve of time to first intravenous device complication per patient (log rank, P = 0.53).
Patient- and device-related variables considered in the multivariable model were older age, number of comorbidities (nil, one, two or more than two), smaller cannula size, poor skin or vein integrity, IV antibiotics, insertion by medical staff and study group. None of these were found to be statistically significant. The final Cox proportional hazards model after adjusting for time found study group was not a significant factor (HR 1.02; 95% CI, 0.77-1.36; P = 0.89). Variance-adjustment testing for potential multiple-failures per patient (cannula data) found no inconsistency in significant results compared to the main Cox model. Developing a Research Base for Intravenous Peripheral Cannula Resites Essay.
