Effect of Solanezumab on Alzheimer’s Disease Essay
Effect of Solanezumab on Alzheimer’s Disease Essay
Alzheimer’s disease is chronic neurodegenerative disease which has a slow development which worsens over a period of time (‘Alzheimer’s Disease’ 2009). This disease is commonly linked with the overabundance of aggregated amyloid-beta (Aβ) peptide within the cerebral cortex and hippocampus (Doody et al. 2014). Solanezumab an Alzheimer’s drug is a monoclonal IgG1 antibody which is used against the mid-domain of the Aβ peptide (Alzforum.org 2015). Effect of Solanezumab on Alzheimer’s Disease Essay. This paper will cover aspects of this drug such as its development, chemical structure and mechanism of action and looking at issues the drug had during its development, regulatory approval and its post market surveillance.
Development
The development of this type of antibody is different from other monoclonal antibodies which are being tested currently. Comparing it to that of another drug called bapineuzumab, which binds to the N-terminal, Solanezumab was created to bind to the soluble Aβ peptide because it was predicted to it being far more effective than binding to the N-terminal portion of a molecule (Imbimbo et al. 2012). In the In Vitro studies which were undertaken for this drug, the parent’s antibody m266 which binds to the Aβ had been tested in a dialysis system involving the antibody solution in the bottom chamber which was divided by a dialysis membrane from the top chamber which had the human CSF. It was seen that a great amount of CSF Aβ was sequestered when the bottom chamber had PBS plus m266 at 48.91% as compared to PBS with a nonspecific mouse IgG being at 2.18% (Imbimbo et al. 2012). The result demonstrated in relation to Aβ binding that m266 was not able to bind to Aβ deposited in parenchyma and cerebral vessels (Imbimbo et al. 2012 cited from [49]).
ORDER A PLAGIARISM-FREE PAPER NOW
Class of drug and Mechanism of action
Solanezumab as defined by the WHO’s International Nonproprietary Names for Pharmaceutical Substances (INN) is an neuroprotectant (WHO 2007). Neuroprotection as dictated by Rafi and Aisen (2009) is the mechanism by which neurons are protected from degeneration; their use can be seen in patients who have suffered recent ischemic injury or a result from neurodegenerative diseases. Effect of Solanezumab on Alzheimer’s Disease Essay.
It is seen that solanezumab’s mechanism of action is different to that of other passive immunotherapies. This is due to it targeting the central domain of Aβ peptide, which has been proposed as more effective in clearing N-terminal truncated or modified forms of Aβ peptide (Siemers et al. 2010). This has separated this drug from others such as bapinezumab which targets the N-terminal of the molecule exclusively (Samadi and Sultzer 2011).
In the murine model, the M266 antibodies are seen to enter the cerebral spinal fluid at a concentration of 0.1% compared that that of in plasma, as with patients with AD, a single injection intravenously of the dose of .5, 1.5, 4 and 10 mg/kg resulted in the maximum plasma concentration for solanezumab (Bruno P Imbimbo, et al. 2012). Furthermore the mean total half-life of the drug was found to be 334 hours (14 days) after an injection of .5mg and 631 to 709 hours (26 to 30 days) when injected with 1.5, 4, or 10 mg which indicated that the lowest dose half-life compared to that of the high doses was most likely due to the drug concentrations falling below quantification limits, thus possibly preventing complete characterization of the terminal elimination phase for the dose given (Imbimbo et al. 2012 cited from Siemers et al. 2010).
Chemical Structure
Solanezumab is a humanized IgG1 derivative of the m266 Aβ monoclonal antibody of a mouse in which binds to the central region of the human Aβ peptide (Stefan Dübel 2014). This antibody was produced inside A/J mice using a synthetic Aβ peptide conjugated with an anti –CD3 immunoglobin (Bruno P Imbimbo, et al. 2012). (Expand a bit more here)
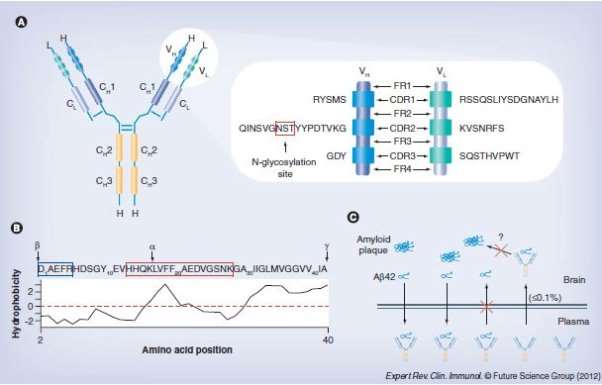
Identifying issues during drug development, regulatory approval or post marketing surveillances
Currently 9 studies have carried out concerning the Solanezumab drug, 5 of which have been completed and the other 4 which are recruiting or currently active in their research (ClinicalTrials.gov 2015). The most well regarded and referenced is the Phase 3 Trials which were carried out by Eli Lilly in 2014 which looked at using Solanezumab for mild to moderate Alzheimer’s disease.
In in murine model of the preclinical trials for the drug were tested for their safety and effectiveness. Effect of Solanezumab on Alzheimer’s Disease Essay.It was seen that the mice treated with the murine version of solanezumab called m266.2 were at risk to develop cerebral microhemorrhaging as compared to mice treated with 3D6 which the bapinezumab model for mice (Samadi, seltzer 2011 cited from [50]). The reasoning for this result was predicted to be that of the different binding paths of the drugs where solanezumab binds to the Aβ peptide exclusively as bapinezumab binds to both Aβ plaque and the n-terminal of the Aβ peptide (Samadi, seltzer 2011 cited from [43]).
Phase I studies showed that when 19 test subjects were subjected to a single dose of solanezumab containing either .5,1.5,4.0 or 10 mg/kg that serious adverse side effects occurred in 4 , 1 in which had a placebo (add in results for this phase 1)( Samadi , Sultzer 2011). The events that occurred was syncope, fatigue and vertigo occurring from the does size given, although it was noted that these effects were not fault of the drug given (Imbimbo et al. 2012 cited [55]). The results of the study had shown that there altogether no changes in the cognitive scores which would indicate that the drug did not provide any benefit.
In the phase II study was conducted looking at the drug being given over a period of 12 weeks.
The various issues that occurred in these studies was that 8 patients had suffered from serious adverse side effects from the drug, these included cardiac, neurological and even gastrointestinal issues (Farlow et al. 2012). The table Figure (1) gives a summary to the adverse events that occurred between the placebo and varying dosage groups of the patients receiving the drug. (expand)
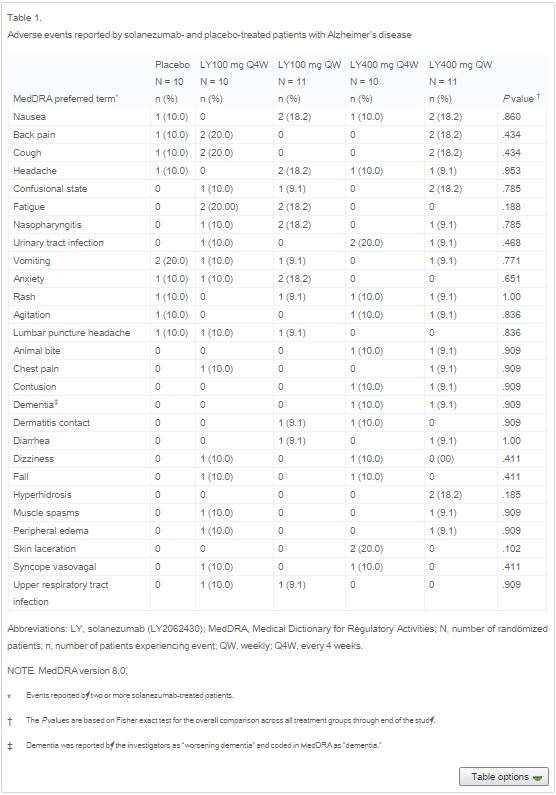
When looking at the cognitive measurements in the patients to assess their cognitive abilities, the results showed that between the drug and the placebo, no important differences were seen on the 11 item or 14 item scores. Effect of Solanezumab on Alzheimer’s Disease Essay. Table figure (3) demonstrates these results showing these differences. This table can then indicate that there was no suggestion that any significant clinical benefit occurred.

The Phase III trials which involved 2 double blind trials which the patients were treated with either the drug or the placebo given periodically over a period of 18 months. The outcomes were measured using the 11 item and 14 items cognitive scores to which the previous phase studies had used.
The results showed that for the baseline characteristics that there were no differences between the control and drug group but for the cognitive and clinical outcomes they
The adverse events that occurred during this trial were that cardiac arrhythmia occurred in 5% of patients who received the drug and 3.7% in the placebo (Doody et al. 2014). As well as the cardiac issues, 33 deaths had occurred, 24 in which were in the group who received Solanezumab
In the discussion section of the studies they mention that from both the studies that were undertaken, none of them had shown any benefit that Solanezumab and the current studies have failed to show treatment effects on the hippocampal , total brain volume or the amyloid accumulation (Doody et al. 2014). Effect of Solanezumab on Alzheimer’s Disease Essay. Doody et al. (2014) goes on to mention that although the study did not show the efficacy of the drug being tested that further studies into the drug will be required to assess the particular approach they’ve taken.
It can be seen a major issue concerning all the studies which were carried out is the lack of efficacy. As can be seen for all the results for the phase studies that all of them showed no significant improvement when it was concerning the 11 item and 14 item scores for the patients.
Conclusion
Solanezumab has also proven to provide a poor efficacy for the patients in which has taken it. The phase studies in which have been reported demonstrate this result occurring which can give evidence toward this particular monoclonal antibody to be ineffective as slowing the progression of Alzheimer’s. Although there was seen adverse side effects in the studies which may call for concern , it has been properly ruled out that the issues were not related to the drug as can be seen in the table results when comparing the control to the drug groups. To summarise solanezumab is not created the same as compared to other monoclonal antibodies, when discussing their binding site on the Aβ peptide, although this drug has proven to have a poor efficacy, it has shown that it causes minimal adverse side effects in comparison to other monoclonal antibodies currently being tested. If more study was to be taken place into altering the drug, in attempt to improve efficacy whilst minimising the adverse effects, it may come into market someday to help people.
References
Bruno P Imbimbo, Simone Ottonello, et al. “Solanezumab for the treatment of mild-to-moderate Alzheimer’s disease.” 2012.
Stefan Dübel, Janice M. Reichert. Handbook of Therapeutic Antibodies. John Wiley & Sons, 2014.
To assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of 12 weekly infusions of solanezumab, an anti-β-amyloid (Aβ) antibody, in patients with mild-to-moderate Alzheimer’s disease. Effect of Solanezumab on Alzheimer’s Disease Essay. Cognitive measures were also obtained. In this phase 2, randomized, double-blind, placebo-controlled clinical trial, 52 patients with Alzheimer’s disease received placebo or antibody (100 mg every 4 weeks, 100 mg weekly, 400 mg every 4 weeks, or 400 mg weekly) for 12 weeks. Safety and biomarker evaluations continued until 1 year after randomization. Both magnetic resonance imaging and cerebrospinal fluid (CSF) examinations were conducted at baseline and after the active treatment period. The Aβ concentrations were measured in plasma and CSF, and the Alzheimer’s Disease Assessment Scale-cognitive portion was administered. Clinical laboratory values, CSF cell counts, and magnetic resonance imaging scans were unchanged by treatment, and no adverse events could be clearly related to antibody administration. Total (bound to antibody and unbound) Aβ(1-40) and Aβ(1-42) in plasma increased in a dose-dependent manner. Antibody treatment similarly increased total Aβ(1-40) and Aβ(1-42) in CSF. For patients taking 400 mg weekly, antibody treatment decreased unbound Aβ(1-40) in CSF (P < .01), but increased unbound Aβ(1-42) in CSF in a dose-dependent manner. The Alzheimer’s Disease Assessment Scale-cognitive portion was unchanged after the 12-week antibody administration. Antibody administration was well tolerated with doses up to 400 mg weekly. The dose-dependent increase in unbound CSF Aβ(1-42) suggests that this antibody may shift Aβ equilibria sufficiently to mobilize Aβ(1-42) from amyloid plaques.
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the most common cause for dementia. There are many hypotheses about AD, including abnormal deposit of amyloid β (Aβ) protein in the extracellular spaces of neurons, formation of twisted fibers of tau proteins inside neurons, cholinergic neuron damage, inflammation, oxidative stress, etc., and many anti-AD drugs based on these hypotheses have been developed. In this review, we will discuss the existing and emerging hypothesis and related therapies.Effect of Solanezumab on Alzheimer’s Disease Essay.
Background
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder, which is the most common cause for dementia and imposes immense suffering on patients and their families. According to the World Alzheimer Report 2016, there are currently about 46.8 million people suffering with AD worldwide. The ageing of world population will further compound this problem and lead to a steep increase in the number of AD patients. The numbers of AD patients are expected to double nearly every 20 years, and thereby the population of AD will reach 74.7 million in 2030 and 131.5 million in 2050 [1]. AD has become the third major cause of disability and death for the elderly, only after cardiovascular and cerebrovascular diseases and malignant tumors.
However, only five drugs have been approved by the FDA to treat AD over the past hundred years since the first AD patient was diagnosed. Not only that, these approved drugs including cholinesterase inhibitors, N-methyl-D-aspartate (NMDA) receptor antagonist or their combination usually provide temporary and incomplete symptomatic relief accompanied with severe side effects. The marginal benefits were unable to slow the progression of AD. Thus, developing drugs for more effective AD treatment is in urgent need.
Current hypothesis about AD and anti-AD drug development
AD is a complicated disease involving many factors. Effect of Solanezumab on Alzheimer’s Disease Essay. Due to the complexity of human brains, the lack of reasonable animal models and research tools, the detailed pathogenesis of AD is still unclear so far. Many hypotheses about AD have been developed, including amyloid β (Aβ), Tau, cholinergic neuron damage and oxidative stress, inflammation, etc. Thus, many efforts have been done to develop anti-AD drugs based on these hypotheses.
Aβ cascade hypothesis
Extracellular deposits of Aβ peptides as senile plaques, intraneuronal neurofibrillary tangles (NFTs), and large-scale neuronal loss were the main pathological features of AD. Thus, Aβ peptides have long been viewed as a potential target for AD which dominated new drug research during the past twenty years [2]. The most direct strategy in anti-Aβ therapy is to reduce Aβ production by targeting β- and γ-secretase [3]. Safety issues are the overriding problem. For targeting γ-secretase, undesirable side effects are inevitable due to its physiological substrates, eg. the Notch signaling protein [4–7], which is essential in normal biological process. Similarily, targeting β-secretase is also challenged for the side effects such as blindness and the large catalytic pocket [8]. More importantly, in sporadic AD cases, the majority of AD patients do not necessarily have over-producted amyloid precursor protein. Besides, Aβ isoforms could also serve as endogenous positive regulators for neurotransmitter release at hippocampal synapses [9]. Thus, inhibiting Aβ production may encounter many challenges.
Aβ clearance by immunotherapy is the alternative choice. For active Aβ-immunotherapy, although the first active AD vaccine (AN1792) developed by ELAN showed some beneficial effects such as less cognitive decline, it was suspended owing to serious side effect, or meningoencephalitis [10–12]. Also, the passive immunotherapy did not do much better than active immunotherapy. Several antibodies targeting Aβ have failed in clinical trials, including bapineuzumab (Pfizer/Johnson & Johnson) [13, 14], Crenezumab (Genentech) [15, 16], solanezumab (Eli Lilly) [16–18] and ponezumab (Johnson & Johnson /Pfizer) [19–21]. Effect of Solanezumab on Alzheimer’s Disease Essay. In addition, although passive immunotherapy could overcome some problems of active immunotherapy, there were still inevitable side effects such as amyloid-related imaging abnormalities [22]. Likewise, the small molecule Aβ binder scyllo-inositol [23] and tramiprosate [24–26] also failed in clinical trials. These failures even cast more doubts on the Aβ theory [27]. Actually, the strategy of targeting only a single functional subregion of Aβ may partly account for these failures [27, 28]. Furthermore, immunotherapy may influence the human immune system, which might cause beneficial or detrimental consequence (such as side effects). However, every cloud has a silver lining. A phase Ib trial of aducanumab (Biogen) showed a positive correlation between brain Aβ levels and disease exacerbation as measured by Clinical Dementia Rating [29–31]. Even the failed phase III EXPEDITION3 trial of solanezumab (Eli Lilly) still demonstrated better performance in Clinical Dementia Rating Sum of Boxes and beneficial impacts on Mini-Mental State Examination and Activities of Daily Living [17, 18, 32, 33]. Thus, despite all kinds of problems, immunotherapy may still be the better approach to modify the extent of neurodegeneration in AD currently [34].
In fact, the original amyloid cascade hypothesis was that “Aβ is the causative agent in Alzheimer’s Disease pathology, and that neurofibrillary tangles, cell loss, vascular damage, and dementia follow as a direct result of this deposition” [35]. After decades of research, although the bulk of data still supports a role for Aβ as the primary initiator of the complex pathogenic cascade in AD, more and more evidences indicate that Aβ acts as a trigger in the early disease process and appears to be necessary but not sufficient in the late stage of AD [36]. Especially, recent rapid progresses in understanding on toxic amyloid assembly and Aβ metabolism associated systemic abnormalities will provide fresh impetus and new opportunities for this interesting approach [37].
Tau hypothesis
Neurofibrillary tangles, another intracellular hallmark of AD, are composed of tau. Tau is a microtubule-associated protein working as scaffolding proteins that are enriched in axons. In pathological conditions, tau aggregation will impair axons of neurons and thus cause neurodegeneration. After numerous failures of Aβ-targeting drugs for AD, more interests are turning to explore the therapeutic potential of targeting tau, particularly as studies of biomarkers suggest that tau pathology is more closely linked to the progression of AD [38]. Effect of Solanezumab on Alzheimer’s Disease Essay.
Tau undergoes many modifications, including phosphorylation, arginine monomethylation, lysine acetylation, lysine monomethylation, lysine dimethylation, lysine ubiquitylation and serine.
O-linked N-acetylglucosamine (O-GlcNAc) modification [39]. Under pathological conditions, increasing of tau hyperphosphorylation will render the protein aggregation-proned, reduce its affinity for microtubules, and thereby influence neuronal plasticity. Consequently, strategies to target tau involve blocking of tau aggregation, utilizing tau vaccinations, stabilizing microtubules, manipulating kinases and phosphatases that govern tau modifications. However, most of these efforts have failed in clinical trials. For Tau aggregation blockers, TRx0237 failed to show treatment benefits in phase III trials [40]. As for vaccinations, tau-targeted active vaccines (ACI35 and AADvac-1) and passive vaccines (RG6100 and ABBv-8E12) are currently in phase I and II clinical trials [41, 42]. Intravenous immunoglobulin (IVIG), the only passive vaccine in phase III clinical trials, failed to meet the primary end points in patients with mild-to-moderate AD [42]. Other tau-targeting strategies for AD, including stabilizing microtubules and manipulating kinases and phosphatases, have just been tested in preclinical studies.
In general, tau-targeting therapies remain challenging because of incomplete understanding of AD, lack of robust and sensitive biomarkers for diagnosis and response-monitoring, and the obstruction of blood-brain barrier.
Inflammation hypothesis
Reactive gliosis and neuroinflammation are hallmarks of AD. Microglia-related pathways were considered to be central to AD risk and pathogenesis, as supported by emerging genetic and transcriptomic studies [43–47]. Increasing evidence demonstrate that microglia emerges as central players in AD. In very early stage, microglia, TREM2 and complement system are responsible for synaptic pruning [48, 49]. The processes of activity-dependent and long-term synaptic plasticity are the common and fundamental cellular underpinning of learning and memory which may manifest as influence on long term potential [50]. Following that, reactive microglia and astrocytes will surround amyloid plaques and secrete numerous pro-inflammatory cytokines. These events are regarded as an early, prime mover in AD evolution. Effect of Solanezumab on Alzheimer’s Disease Essay. However, non-steroid anti-inflammatory drugs (NSAIDs) did not show enough benefits in clinic. This is because that the relationship between innate immunity and AD pathogenesis is complex, and the immune response can be either deleterious or beneficial depending on the context [47, 51, 52]. However, the new observations that PD-1 immune checkpoint blockade reduces the pathology of AD and improves memory in mouse models of AD [53–55] give us a direction of future researches.
The recent advances in our understanding of the mechanism underlying microglia dysfunction in pruning, regulating plasticity, and neurogenesis are opening up possibilities for new opportunities of AD therapeutic interventions and diagnosis [56, 57]. Targeting these aberrant microglial functions and thereby returning homeostasis may yield novel paradigms for AD therapies. However, given the complexity and diverse functions of microglia in health and disease, there is a crucial need for new biomarkers reflecting the function of specific microglias [52, 58].
Cholinergic and oxidative stress hypothesis
Acetylcholine (ACh) is an important neurotransmitter used by cholinergic neurons, which has been involved in critical physiological processes, such as attention, learning, memory, stress response, wakefulness and sleep, and sensory information [59–63]. Cholinergic neurons damage was considered to be a critical pathological change that correlated with cognitive impairment in AD. Thus, cholinergic hypothesis was firstly tested with cholinesterase inhibitors in AD treatment. Tacrine, a cholinesterase inhibitor, was the first anti-AD drug available in clinic [64–66] although it was withdrawn from the market in 2012 due to severe side effects. Although inhibiting cholinesterase is a symptomatic relief treatment with marginal benefits, it is currently the most available clinical treatment which gives desperate AD patients a glimmer of hope. For other neurotransmitter dysfunction, such as Dopamine and 5-hydroxytryptamine, there are some studies about them, but not much as acetylcholine in AD. Effect of Solanezumab on Alzheimer’s Disease Essay.
Oxidative stress is considered to play an important role in the pathogenesis of AD. Especially, the brain utilizes more oxygen than other tissues and undergoes mitochondrial respiration, which increases the potential for ROS exposure. In fact, AD is highly associated with cellular oxidative stress, including augmentation of protein oxidation, protein nitration, glycoloxidation and lipid peroxidation as well as accumulation of Aβ, for Aβ can also induce oxidative stress [67–73]. Thus, the treatment with anti-oxidant compounds would provide protection against oxidative stress and Aβ toxicity in theory. However, oxidative stress is only a single feature of AD, so antioxidant strategy was challenged for its potency to stop the progression of AD and thus it is proposed as a portion of combination therapy [74, 75].
Glucose hypometabolism
Glucose hypometabolism is the early pathogenic event in the prodromal phase of AD, and associated with cognitive and functional decline. Early therapeutic intervention before the irreversible degeneration has become a consensus in AD treatment. Thus, alleviation of glucose hypometabolism was emerged as an attractive strategy of AD treatment. However, most of these therapeutic strategies are targeting mitochondria and bioenergetics, which have shown promise at the preclinical stage but without success in clinical trials [76, 77]. Although no strategies are available to alleviate glucose hypometabolism in clinical, glucose metabolism brain imaging such as 18FDG-PET (Positron emision tomography with 2-deoxy-2-fluorine-18-fluoro-D-glucose) has become a valuable indicator for diagnosis of neurodegenerative diseases that cause dementia, including AD [78].
Up to now, there’re no effective treatments for changing the course of AD. Confronting these difficulties, we should get deeper understandings about these hypotheses, and meanwhile we should renovate our knowledge about AD and develop new hypothesis.
New pathway to AD
AD is conventionally regarded as a central nervous system (CNS) disorder. However, increasing experimental, epidemiological and clinical evidences have suggested that manifestations of AD extend beyond the brain.Effect of Solanezumab on Alzheimer’s Disease Essay. Most notably, research over the past few years reveals that the gut microbiome (GMB) has a profound impact on the formation of the blood-brain barrier, myelination, neurogenesis, and microglia maturation [79–84]. In particular, results from germ-free animals and animals exposed to pathogenic microbial infections, antibiotics, probiotics, or fecal microbiota transplantation showed that gut microbiota modulates many aspects of animal behaviors, suggesting a role for the gut microbiota in host cognition or AD-related pathogenesis [85–88]. The underlying mechanisms of gut microbiota influencing brain involve the communication through immune system, the endocrine system, the vague nerve, and the bacteria-derived metabolites.
Immune pathway
The intestinal mucosal lymphoid tissue contains 70% ~ 80% of the immune cells in the whole body, and is considered to be the largest and most important human immune organs. It is also the first line of host defense against pathogens. The human gut contains a large, diverse and dynamic enteric microbiota, including more than 100 trillion microorganisms from at least 1000 distinct species. There’s a complex relationship between intestinal mucosal immune system and intestinal microbiota. Thus, gut microbiota induced immunomodulation is emerging as an important pathway that influences AD [89].
Gut microbiota can influence brain through immune system in several ways. Firstly, intestinal microbiome can induce cytokines secretion, which enter the circulatory system, pass through blood brain barrier, and directly affect the brain function. For instance, perivascular macrophages and cerebral small vessel epithelial cells can receive the intestinal microbiome produced IL-1 signal and affect central nervous system. Also, gut microbes can activate Toll-like receptors of the brain immune cells (such as microglia) through microbes associated molecular patterns (MAMP).Effect of Solanezumab on Alzheimer’s Disease Essay. MAMPs can either directly bind to intestinal epithelial cells or infiltrate to the intestine lamina propria to activate lymphocytes, promoting the release of pro-inflammatory cytokines, which further cause subsequent inflammation in brain. Secondly, gut microbes can produce metabolites such as short-chain-fatty acids (SCFAs), gamma-aminobutyric acid (GABA) and 5-HT precursors, which could also travel to the brain via circulatory systems or signal through intestinal epithelials to produce cytokines or neurotransmitters that activate vagus nerve. Thirdly, gut microbes can activate enteroendocrine cells to produce 5-HT, which affect the brain through neuroimmune pathways.
In addition to changing the functions of the immune system, such as through secretion of inflammatory factors or anti-inflammatory factors, intestinal microbiome can also affect the development and composition of immune system. For example, in germ-free mice, isolated lymphoid follicles in gut associated lymphoid tissue are unable to mature, and lymphocytes that are able to secrete IgA in the intestinal epithelium decreased [89–92]. For immune system in brain, the deletion of gut microbiota in germ-free mice have global influence on the cell proportions and maturation of microglia in the brain, and thus affect the properties and phenotype of microglia, as compared to conventionally colonized controls [93]. Similar results were obtained in antibiotic treated mice. Other research also demonstrates that the number of T regulatory cells and T helper lymphocytes (T helper 17, Th17) are significantly reduced in the germ free mouse, indicating the regulatory effects of intestinal microbiome on T cell composition, while microbiome tansplant to germ free mice can modify these variations and restore normal immune function [94, 95]. All these modulations of gut microbiota may have direct and indirect effects on AD development and progression.
Endocrine pathway and the vagus nerve
The gut is also the largest endocrine organ in the body. Gut microbiota can regulate secretion of many hormones from intestinal endocrine cells, such as corticosterone and adrenal hormones, and thus establish the information exchange between the intestines and the brain. For example, the intestinal microbiome can affect the secretion of serotonin and regulate brain emotional activities [96, 97]; intestinal microbial metabolism can also produce a variety of neurotransmitters, such as dopamine, GABA, acetylcholine and melatonin, which are transmitted to central nervous system through the vagus nerve [98]. Effect of Solanezumab on Alzheimer’s Disease Essay. Besides transporting these signal substances, the vagus nerve itself plays an important role in inflammation and depression [99]. The vagus nerve can influence the gastrointestinal tract, orchestrate the complex interactions between central and peripheral neural control mechanisms [100]. The stimulation of vagus nerve is able to regulate mood, and the immune system, suggesting the therapeutic potential of vagus nerve modulation to attenuate the pathophysiological changes and restore homeostasis [98–103].
Bacteria-derived metabolites
Generation of essential nutrients for host physiology, such as vitamins and other cofactors, is an important physiological function of the gut microbiota [104]. The metabolites of microbiome, such as SCFAs including acetate, butyrate, and propionate, are able to modulate peripheral and central pathologic processes [105]. For example, butyrate is effective in reducing inflammation and pain. Once in the brain, acetate is able to alter the level of the neurotransmitters glutamate, glutamine, and GABA, as well as increases anorectic neuropeptide expression [106]. In addition, the gut microbiota can secrete large amounts of amyloids and lipopolysaccharides, which might contribute to the modulation of signaling pathways, the production of proinflammatory cytokines associated with AD pathogenesis and Aβ deposition [107–109].
ORDER A PLAGIARISM-FREE PAPER NOW
In fact, microbiota-gut-brain axis has been established and a disturbed gut microbiota has been incriminated in many neurodegenerative diseases in animal and translational models. In theory, a role for the microbiota-gut-brain axis is highly plausible. However, the theoretical basis for the use of microbiota-directed therapies in neurodegenerative disorders still needs supports from high-quality clinical trials [110]. To date, only a few studies directly focused on the gut microbiota and AD [111, 112], and studies on AD patients is particullarly deficient. A recent research from human showed an increase in the abundance of a pro-inflammatory GMB taxon and a reduction in the abundance of an anti-inflammatory taxon are possibly associated with a peripheral inflammatory state in patients with cognitive impairment and brain amyloidosis. It is important for the research of gut microbiota and AD. Effect of Solanezumab on Alzheimer’s Disease Essay. However, further investigations are still necessary to explore the possible causal relation between GMB-related inflammation and amyloidosis [111]. The comprehensive understanding of these underlying mechanisms may provide new insights into these novel therapeutic strategies for AD. In particular, based on the gut microbiota hypothesis, Chinese traditional medicine and probiotic bacteria may play a more important role in therapy [113].
Conclusions
Nowadays, new technologies are making it possible to get to know enough pathologic details of disease. More importantly, scientists are beginning to treat AD as a systemic disease and they are paying more attention to the correlation between brain and other organs [47, 89, 114]. Perhaps, for complicated disease such as AD, researches and therapies should be based on the principle that combined reductionism with holism, and great efforts should be made to search the fundamental laws of AD by means of multi-scale modeling and efficient numeric assessment. Maybe, just like Chinese traditional medicine [115], combination treatments or systematic therapy will be a final way out. Effect of Solanezumab on Alzheimer’s Disease Essay.
