Managing the Risk of Opioid Prescription Essay
Managing the Risk of Opioid Prescription Essay
Instructions: After viewing this week’s Pri-Med lecture, write 2–3 paragraphs on how you will incorporate this information into your practice. Include a reference in APA style. Managing the Risk of Opioid Prescription As a family nurse practitioner, there is a responsibility to manage the risk of opioid prescriptions since more than 50% of opioid prescriptions are done by primary care providers and certified nurse practitioners. According to Hudspeth (2016), the first two components of opioid prescriptions are the history of the patient and assessment. I would use the information to carry a thorough medical background check on the patient to familiarize myself with the patient’s history of opioid-related issues such as cognitive impairments and renal diseases.
Years of sustained, coordinated, and vigilant effort will be required to contain the present opioid epidemic and ameliorate its harmful effects on society. Managing the Risk of Opioid Prescription Essay. At least 2 million people have an opioid use disorder (OUD) involving prescription opioids, and almost 600,000 have an OUD associated with heroin (HHS, 2016). These numbers are likely to increase in the coming years, regardless of what policies are put in place. Follow-up studies of individuals receiving treatment for OUD involving heroin (e.g., Hser et al., 2001) find very high rates of premature mortality (in the neighborhood of one-third) due to overdose or other complications of the disorder. Thus, even if the nation ramps up treatment availability substantially and immediately, death rates will climb and quality of life will be dramatically reduced for many people for years to come. Likewise, the continued progression of still more people from prescription opioid use to OUD will demand sustained and coordinated effort to establish and implement the scientifically grounded policies and clinical practices necessary to reshape prescribing practices and reduce the occurrence of new cases of prescription opioid-induced OUD.1
What should be done to contain the opioid epidemic and to prevent new cases of iatrogenic addiction and associated overdose, death, and other harms? The purpose of this chapter is to review available evidence on strategies that have been used to address the problems of opioid misuse, OUD, and related deaths. The chapter begins with prefatory sections addressing (1) the nature of the evidence on policies implemented at the jurisdictional level (typically a state or a nation), as opposed to clinical interventions operating at the level of an individual patient; and (2) the need for a systems approach, including the importance of recognizing the potential effects that interventions focused on misuse of prescription opioids have on misuse of opioids more generally. Next the chapter reviews the evidence on the effectiveness of strategies for addressing the opioid epidemic in four categories: (1) restricting supply, such as by regulating the types of products approved for use (e.g., abuse-deterrent opioids) and regulating/restricting conditions of lawful access to approved drugs; (2) influencing prescribing practices, such as through provider education and the issuance of prescribing guidelines; (3) reducing demand, such as by educating patients about opioids and increasing access to treatment for OUD; and (4) reducing harm, such as through provision of naloxone to prevent opioid overdose and needle exchange programs for people who use injection drugs. Managing the Risk of Opioid Prescription Essay.
ORDER A PLAGIARISM-FREE PAPER NOW
NATURE OF THE EVIDENCE
Theoretically, the comparative effectiveness of different opioid-related policies could be quantified through use of randomized controlled trials (RCTs). For example, consider a clinical strategy that eschews prescribing opioids to treat chronic noncancer pain if the patient scores high on a scale used to measure risk of developing opioid addiction. The effectiveness of this strategy for preventing OUD could be evaluated in an RCT in which patients were assigned to either that policy intervention or an alternative one with fewer restrictions on opioid prescription. An RCT is the preferred source of evidence for causal inference because the random assignment is expected to result in comparable groups of individuals assigned to each strategy. In a large RCT of different approaches to opioid prescribing for preventing OUD, for example, one would expect patients in each group to have, on average, the same risk factors for developing OUD. Managing the Risk of Opioid Prescription Essay. That is, any future differences between the groups in the frequency of OUD could be ascribed to the different treatment strategies to which they were assigned rather than to differences in the characteristics of the individuals receiving each strategy. As a result, the outcome distribution in each group could be interpreted as the counterfactual outcome distribution that would have been observed in that population under the corresponding strategy.2
RCTs, however, are rare for policies that require implementation at the level of an entire jurisdiction, nor are they ethically permissible in many policy contexts. In the absence of RCTs, other sources of evidence are needed to estimate the counterfactual outcome distribution under different strategies. One such source of evidence is the collection of data on individuals who happen to receive the strategies of interest as part of their routine care, often from electronic health records. The so-called observational analyses based on such data are attempts to emulate the RCT that cannot be conducted (the target trial). In these observational analyses, however, the comparability of the groups receiving each strategy is not guaranteed. In the real world, for example, the restricted opioid prescription policy might more likely be applied to individuals visiting providers in urban health care settings who also received other interventions to reduce the risk of addiction. As a result, a direct comparison of the outcome distribution between those who received each strategy would be confounded by the concomitant interventions.
Observational analyses attempt to eliminate bias due to confounding by adjusting for all measured prognostic factors that are distributed differentially between the groups. For example, the comparison might be conducted separately among individuals in urban and rural health care settings. If all confounding factors are appropriately measured and adjusted for, the observational analysis will adequately emulate the target trial and correctly estimate the counterfactual scenarios under each strategy. But even if confounding is eliminated in an observational analysis, this source of evidence is inherently limited with respect to the counterfactual scenarios it can recreate. Managing the Risk of Opioid Prescription Essay. Analyses of observational data may be helpful for estimating the comparative effects of different treatment strategies applied to a clinical population, but may not capture population-level effects under different policies. For example, an observational analysis of patients of certain health care providers will not quantify effects due to scaling up a treatment strategy as a policy applied to the entire health system.
In fact, this chapter typically investigates the effects of strategies that operate at the level of a jurisdiction, such as a locality or state, or that of the country as a whole. Because random assignment is exceedingly rare in such circumstances (no one, for example, is authorized to randomly assign New Hampshire and 24 other states to receive one policy or to freeze policy in the other 25 states so they can serve well as controls), and observational analyses of clinical populations cannot capture system-wide effects (even if they could successfully adjust for confounding), other approaches are needed. All of these approaches will lack physical randomization of the strategies being examined and therefore will be subject to confounding, but they nonetheless are essential sources of evidence for estimating the effectiveness of various strategies.
Before–After Comparisons
A common nonrandomized source of evidence is before–after comparisons, or the comparison of population outcomes before and after a strategy has been implemented in a single population. Because of underlying trends, however, this comparison may provide a biased estimation of the counterfactual scenarios. For example, the strategy might have been implemented in a population precisely because conditions in that population had been deteriorating. If the underlying factors that gave rise to this trend persisted, conditions might continue to worsen after the strategy was implemented even if the strategy was helpful because it diminished but did not reverse the rate of deterioration. Or the implementation process might move so slowly that the strategy did not take effect until the underlying problem had already exhausted its momentum, and a sort of regression to the mean thus created the illusion that the policy was more effective than it truly was. Therefore, a before–after comparison may not correctly identify the counterfactual of how the world would have looked in the absence of the strategy’s implementation. Managing the Risk of Opioid Prescription Essay.
Ecological Comparisons
Another nonrandomized source of evidence is ecological comparisons, or comparison of outcomes between two different populations, only one of which has received the strategy. Again, however, this comparison may provide a biased estimation of the counterfactual scenarios because the policy may have been implemented in one of the populations precisely because conditions had been deteriorating, or other important between-population differences in prognostic factors may have affected the outcome.
An additional challenge for nonrandomized sources of evidence is that many strategies may exert effects that extend across jurisdictional boundaries or manifest only with a considerable lag. For example, even a successful intervention might noticeably reduce the incidence of overdose only many years after being implemented. Indeed, some interventions that successfully reduced diversion of prescription opioids might, at least in theory, initially increase rather than decrease the number of overdose deaths, even if they reduced deaths in the long run, as the result of an initial surge in deaths among people already addicted to prescription opioids who turned to black market substitutes, whose potency is more variable. Furthermore, some interventions may have different effects depending on the metric employed; thus, for example, distributing naloxone might reduce the number of fatal overdoses but—particularly if there were some risk compensation or other behavioral adaptation—increase the total number of overdose events. Strang and colleagues (1999), for instance, found that 6 percent of individuals in treatment for opioid addiction who were interviewed (9 of 142) reported that access to naloxone might lead them to increase their heroin dosage.
Another problem is that of nonlinear response in systems that have their own internal dynamics. For example, resale or other diversion of prescription opioids by people who had already “traded down” to cheaper black market opioids might cause others to initiate misuse of prescription opioids, others who themselves might later trade down, divert, and supply still others. Managing the Risk of Opioid Prescription Essay. This problem is illustrated by the difficulty of talking about the number of cases of an infectious disease that are prevented per vaccination as if it were a universal constant, whereas that number in fact depends on the number of other vaccinations being given and the current prevalence of the disease.
THE NEED FOR A SYSTEMS APPROACH
A complementary approach to evaluating intervention strategies implemented at the jurisdictional level in systems with lags and nonlinearities is to use some model of the system in question to project what might be expected with and without the intervention of interest. This approach has been used in a variety of contexts, including air traffic control (Bertsimas and Patterson, 1998; Long et al., 1999; Terrab and Odoni, 1993), fisheries management (Bjørndal et al., 2004; Clark, 1990; Megrey, 1988), vaccination (Goldstein et al., 2005; Kaplan et al., 2002; Medlock and Galvani, 2009), and tobacco control (IOM, 2007, 2015; Levy et al., 2005), among many other important policy domains.
The dynamics of prescription opioid misuse are complicated, particularly when one takes into account the markets for diverted and purely illegal opioids, but a simple sketch helps clarify the value of a systems approach. A typical clinical trajectory that policy changes would like to prevent starts with medically appropriate use of prescription opioids, escalates to misuse and then to OUD, and then evolves to trading down to cheaper black market opioids before manifesting in overdose. Managing the Risk of Opioid Prescription Essay. Thus, a leaky prescription drug system increases the flow of people into the state of having OUD. People tend to remain in that state for a very long time, an average of 10 to 20 years, with modest flows out of that state through overdose death, death from other causes, or permanent cessation of use.3
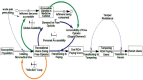
FIGURE 5-1
A systems model of the opioid misuse problem. SOURCE: Reprinted from Wakeland et al., 2015.
The number of overdoses per year might be roughly proportional to the number of people who currently had an active OUD, but this number would not be proportional to the current inflow of new people developing OUD, which is what many interventions aimed at controlling the misuse of prescription opioids would affect most directly. Those interventions would not instantly change the prevalence of OUD and hence would generally not have an immediate effect on overdose. By contrast, interventions that reduced the likelihood that an overdose would occur, or that it would be fatal, might reduce fatalities right away. Managing the Risk of Opioid Prescription Essay.A fair comparison of the effectiveness of interventions designed to reduce diversion with those designed to reduce the frequency or lethality of overdoses requires a true systems model, not just simple statistics. Wakeland and colleagues (2015) provide an example of such a systems model, reproduced in Figure 5-1.
Constructing such models is a major research endeavor in its own right, and the committee is unaware of any existing model that incorporates all of the strategies discussed in this chapter; therefore, the relative effectiveness of these strategies cannot be compared. Creating such models would have important advantages: it would guide and strengthen surveillance and research, foster a common policy vocabulary among all agencies with decision-making authority over opioid regulation and enforcement (federal, state, and local), and facilitate the exchange of information among them. Investing in research and possible development of such a model is worthy of consideration by the U.S. Food and Drug Administration (FDA) and other agencies. In any event, since no formal systems model now exists, the committee provides an overview of the key conceptual features and implications of a systems approach (without a formal model) to identify some of the considerations that need to be taken into account in reviewing the possible impact of alternative strategies. However, empirical analysis of the various strategies reviewed in this chapter relies on the traditional statistical methods outlined in the previous section.
A Systems Approach to Opioid Misuse
The boundaries delineating governmental agencies’ respective responsibilities do not always align with the real boundaries of markets or behaviors concerning OUD and resulting overdose. While the FDA’s regulatory authority may give it a particular interest in reducing addiction and mortality caused by prescription opioids, the nation’s overall public health interest lies in reducing addiction and mortality caused by opioids of all sorts. A person with prescription opioid–related OUD may escalate his or her opioid misuse, and an overdose leaves a grieving family wondering whether or not the person’s last dose was obtained through a prescription.
Prescription and nonprescription opioids intertwine on both the demand and supply sides of the market because all opioids belong to one family of chemicals that operate on similar molecular pathways; the molecules bind to a neuroreceptor regardless of whether they are associated with a prescription. In addition, as shown in Chapter 4, the prescription opioid epidemic is interwoven with the illegal drug market. Managing the Risk of Opioid Prescription Essay. Therefore, this chapter considers policy options for reducing OUD, mortality due to opioid overdose, and other opioid-related harms among people who have ever used prescription opioids, rather than focusing exclusively on options for reducing misuse of or overdoses from prescription opioids alone.
In the economic sense of the term, all opioids are substitutes (as opposed to complements) in the same sense that oil, gas, coal, nuclear, solar, and hydro are substitute sources of energy for producing electric power. Substitutes are not identical and interchangeable; a molecule of morphine is different from a molecule of fentanyl, just as a barrel of oil differs from a ton of coal. There are distinguishable groupings within broad families of substitutes. Energy policy distinguishes fossil fuels from sources with lower carbon footprints; in this context, one can distinguish partial from complete opioid agonists. But just as one cannot develop a sensible response to global warming by changing only policies toward oil, one cannot develop a sensible response to the nation’s opioid problem by adjusting only policies concerning prescription opioids.
The central economic idea about substitutes is that people will tend to use more of item A and less of item B when the price of A falls relative to the price of B, where price is construed broadly to mean the total cost of obtaining and using the item. For opioids, that total cost includes not only the dollar price, but also the time and inconvenience of obtaining the drug and all relevant risks in terms of health and possible criminal justice sanctioning (Moore, 2013; Reuter and Kleiman, 1986; Rocheleau and Boyum, 1994). A related concept is substitution driven by changes in income; as people become poorer, they may substitute hamburger in place of steak and heroin in place of prescription opioids (Petry and Bickel, 1998).
As noted earlier and discussed in greater depth in Chapter 4, in the case of the opioid epidemic, one common pathway to death over the past 20 years has been becoming addicted to prescription opioids, no longer being able to sustain that habit financially, and so trading down to cheaper black market opioids before dying of an overdose or suicide. Trading down can also involve beginning to inject drugs, since that is a more efficient mode of ingesting psychoactive substances. Therefore, additional opioid-relevant public health outcomes include morbidity and mortality stemming from bloodborne infection (e.g., hepatitis C virus [HCV], HIV), both for the individuals injecting and for others (e.g., sexual partners). These outcomes remain relevant even if, for example, no prescription opioids were taken during the month preceding death due to AIDS. Managing the Risk of Opioid Prescription Essay.
Conversely, finding large amounts of a prescription opioid in the decedent’s body does not imply that the person had a prescription. It is common for people who have traded down to black market drugs to retain their prescriptions for purposes of reselling those drugs on the black market. In 2016, typical street prices were $10–$30 for a 30 mg tablet of oxycodone, $5–$20 for a methadone tablet, $3–$8 for Vicodin, and $1 per mcg per hour for fentanyl patches (WSIN, 2016). Thus, diverting to the black market a prescription for two 30 mg tablets per day can produce revenues of $7,300–$21,900 over the course of 1 year. That income is tax-free and mostly pure profit because the copays for those prescriptions are typically small, as is the case for those filled through Medicaid, for example.
Thinking beyond prescription-related misuse becomes all the more important when one recognizes that the same chemicals that appear in prescription drugs are increasingly reaching users not only through diversion but also via distribution chains that are illegal from top to bottom. So even when an autopsy shows that the decedent’s body contained a drug that is available by prescription, this does not mean that the fatal dose was obtained through a prescription by the decedent or anyone else.
In particular, drug trafficking organizations increasingly use fentanyl to adulterate black market heroin and counterfeit pills that have been stamped to look like prescription drugs. This black market fentanyl is produced in the same countries—perhaps even in the same laboratories—that sell fentanyl to pharmaceutical companies that supply prescription fentanyl in lozenges and transdermal patches. Likewise, the pill presses and dyes that these firms sell to the drug trafficking organizations that press the powdered fentanyl into counterfeit tablets of opioid painkillers (e.g., oxycodone) and benzodiazepines in North America are the same as those used by other firms to make the tablets sold to the pharmaceutical companies (DEA, 2016a, p. 7). Thus, not only is black market fentanyl the same chemical compound as pharmaceutical fentanyl, but it may even have the same provenance. That in turn means there is no practical way to count precisely how many overdose deaths are due to prescription opioids even in the narrow sense that the proximate cause of death was a dose that had been prescribed.
It is worth noting that black market fentanyl is a relatively recent phenomenon. Managing the Risk of Opioid Prescription Essay. Until 2014, the number of fentanyl exhibits reported by the National Forensic Laboratory Information System (NFLIS) remained below 1,000, except for a spike to 1,594 in 2006, when a single clandestine lab in Toluca, Mexico, fueled the fentanyl outbreak. The number of exhibits soared in 2014, accompanied by sharp increases in deaths despite no comparable increase in prescribing (Gladden et al., 2016), and reached 13,002 in 2015 (DEA, 2016a).
Price data suggest this trend may continue to intensify. The U.S. Drug Enforcement Administration (DEA) reports that traffickers can buy powdered fentanyl from suppliers for a few thousand dollars per kilogram when buying in bulk (e.g., 20 or 40 kg lots) (DEA, 2016a). Since a counterfeit tablet contains only about 0.9–6.9 mg of fentanyl, the active ingredient can cost high-level traffickers just a penny or two for a pill that wholesales for $6.50 and retails on the street for $10–$20. By comparison, over the past decade, black market retail prices were roughly $500 for a gram of powder 30 percent heroin by weight. So while black market heroin has been much less expensive than (real) diverted prescription opioids, fentanyl is now much less expensive per morphine-equivalent dose than has been the case for black market heroin.
Drug markets are often characterized by substantial price increases as one moves down the distribution chain, but in the case of opioids these increases can be comparatively extreme (in some locations) (Caulkins et al., 2016), which suggests that the current price structure is unstable (Caulkins et al., 2016; Reuter and Kleiman, 1986). The situation is unprecedented, so it is difficult to know how it will develop, but it would not be entirely surprising if the market for counterfeit prescription pills were to undermine the market for real prescription pills. Should this occur, it might reduce the prescription drug overdose problem in its narrowest form, but it would not decrease the total number of opioid-related deaths.
The desire to root opioid policy making in an integrated systems perspective has three corollaries that bear discussion: (1) an ongoing research program is needed to continuously improve understanding of how the various opioids in all their combinations are used and misused in fact, as opposed to just as intended; (2) investment is warranted in an underlying data infrastructure, as opposed to piecemeal efforts local to particular considerations; and (3) the capability to monitor, understand, and model that behavior can be shared among all agencies that have decision-making authority over opioid policy (federal, state, and local), as not all agencies can or should invest in model building within their own silos. Managing the Risk of Opioid Prescription Essay.
Need for a Formal Quantitative Model
Ideally, an integrated framework for regulatory decision making, discussed further in Chapter 6, would rely on an explicit model of the opioid ecosystem. This is because, as discussed above, decisions made about complex systems with endogenous feedback can be myopic in the absence of a formal model. It would be sensible for the FDA, in collaboration with the U.S. Centers for Disease Control and Prevention (CDC), to commission a panel of experts to develop a quantitative model of prescribed and illicit opioid use and distribution and establish the data infrastructure needed to support and apply that model. With such a model, the FDA and other government agencies could predict the effects of changes in policy or other changes in the opioid ecosystem.
If a model capturing the relevant outcomes in the opioid ecosystem were to be developed, that effort would not be accomplished overnight. The process would take time, and important decisions regarding opioids would have to be made in the interim. For now, then, agencies will need to integrate and weigh data from multiple sources and consider the multiple complex feedback processes without the benefit of a formal model. In Chapter 6, the committee outlines some key attributes of any sound framework for decision making involving opioid regulation. At the very least, these attributes will help in making judgments transparent, highlighting areas of uncertainty and the nature of the qualitative judgments that were made.
In sum, when evaluating past policies and estimating the effects of future interventions, it is necessary to use a comprehensive approach that takes full account of the interactions between prescription and black market opioids. Ideally, this approach could take the form of a quantitative model, although developing such a model would itself be an ambitious research undertaking.
Categorizing Strategies for Addressing the Opioid Epidemic
In traditional policy discourse relating to use of addictive drugs, analysts typically categorize available strategies (including specific policies and interventions) as aiming either to (1) reduce supply or the availability of the addictive drug, (2) reduce demand for the addictive drug, or (3) reduce the likelihood that use of the drug will have harmful consequences (see Box 5-1 for a list of strategies discussed in this chapter). Managing the Risk of Opioid Prescription Essay. Like all typologies, this one presents challenges of classification, but it will serve well enough in the present context by enabling the committee to summarize the evidence on the effectiveness of the wide range of policies and interventions now being deployed to address the opioid epidemic.
Several preliminary observations are necessary to avoid misunderstanding. First, each strategy has its own costs and entails trade-offs. Obviously, one of the key trade-offs at the heart of this report is the tension between reducing the supply of opioids to reduce harms associated with their misuse and making opioids available to provide pain relief for individuals who have no satisfactory alternative. Second, strategies cannot be fully evaluated in isolation from one another. Sometimes they are seen, mistakenly, to be in tension with one another, as in the example that making naloxone available to prevent a fatal overdose (harm reduction) can counteract policies aiming to discourage opioid misuse. In other cases, different strategies may have additive effects or even potentiate one another, such that each is stronger and more effective than it otherwise would have been; for example, some observers have pointed out that one way in which some tobacco control interventions are effective is through synergy of multiple intervention components (Green and Kreuter, 2010). In still other cases, successful implementation of some strategies (and the effectiveness of a jurisdiction’s overall approach) may require that strategies be implemented in tandem with one another. A good example is that a strictly enforced supply reduction strategy may cause substantial harms to individuals with OUD (and to society) unless treatment opportunities are aggressively increased.
Finally, it is important to note that very little research has addressed the relationship among strategies. Thus, strategies A, B, and C may each have a small effect, but what would happen if they were all implemented simultaneously and vigorously is unknown. This limitation is critically important in the context of this report.Managing the Risk of Opioid Prescription Essay. The data reviewed in this chapter suggest that many strategies might each have a small effect in reducing opioid misuse and related harms, but simultaneous and vigorous implementation of all of these strategies would still leave a huge reservoir of people misusing and addicted to opioids for years if not decades to come.
BOX 5-1
Strategies for Addressing the Opioid Epidemic.
Another important point to make at the outset is that the strategies reviewed in this chapter have been adopted and implemented by a wide variety of public and private entities at the national, state, and local levels. The literature reviewed in this chapter demonstrates that there is currently no national strategy. Nor is there a lead agency responsible for crafting and implementing such a strategy or integrating efforts across levels of government (local, state, or national). While formulating a national strategy and suggesting which agencies should implement it are beyond this committee’s charge, this approach is worthy of consideration.
STRATEGIES FOR RESTRICTING SUPPLY
As discussed previously, the responsible clinical use of prescription opioids can be a powerful tool for pain management under some circumstances. The primary area of continuing concern relates to long-term use of opioids to alleviate chronic noncancer pain. A constellation of policies related to lawful access and judicious clinical decision making can help ensure that opioid-related harms are minimized while providing access to these drugs for patients with appropriate clinical indications. This section reviews such supply-side strategies, including regulation of legal access to opioids for legally approved uses. The next section addresses legal regulations and professional policies aimed at reducing lawful access by discouraging unnecessary opioid prescribing or promoting safe prescribing practices. Although both types of strategies aim to control access to opioids, the former focuses on legal restrictions on distribution, while the latter focuses on efforts to influence the decisions of health care providers as the gatekeepers to lawful access by patients.
Regulating the Approved Product: Abuse-Deterrent Opioids as a Case Study
The FDA’s decision to approve a new drug follows a rigorous review of product- and indication-specific benefits and risks. In the case of opioids, a drug is reviewed for its ability to provide analgesia, weighed against the potential risk of adverse effects (e.g., dependence, addiction, nausea and other side effects to the patient). Often, the benefit calculus includes product-specific features, such as high-dose extended-release (ER) formulations for pain that is long-lasting and especially severe. Managing the Risk of Opioid Prescription Essay.The drug is then ultimately approved for use in a specific population for a specific clinical indication, based on the totality of evidence considered by the FDA for that particular population and indication (see Chapter 6 for a suggested approach for FDA decision making on and post-market monitoring of opioids).
However, one consequence of early ER opioid formulations was unexpectedly high misuse. In response, a new product feature—designated abuse-deterrent formulations (ADFs)—has been a focus of FDA policy for addressing the opioid epidemic. ADFs are opioid medications that have been reformulated to reduce the possibility or the likelihood that the medication will be “abused.” While users may misuse opioid medications by swallowing pills whole, the misuse often involves manipulation of the pills. For example, a user may crush the pill and then swallow, snort, or smoke it, or dissolve and inject it. Many ADFs are designed to discourage manipulation either by making the pill difficult to manipulate or by rendering it ineffective or unpleasant once manipulated. Abuse-deterrent technologies include the following (FDA, 2015a):
-
Physical designs that are crush/extraction-resistant—For example, OxyContin, a form of ER oxycodone, incorporates a hard polymer matrix that makes crushing or chewing the pill difficult and that transforms into a viscous gel when dissolved in water (which prevents extraction). Formulations that integrate such physical barriers often are referred to as “tamper-resistant opioids.”
-
Chemical barriers that prevent extraction of the opioid with solvents.
-
Agonist/antagonist combinations that interfere with the euphoria associated with use of opioids—These ADFs include coformulations of opioids with sequestered naltrexone or naloxone. Inadequate pain relief and even acute opioid withdrawal are concerns with the use of these formulations.
-
Aversion formulations that include a substance that produces an unpleasant effect if the medication is misused.
-
Delivery systems that are resistant to “abuse,” such as subcutaneous implants. Managing the Risk of Opioid Prescription Essay.
-
New molecular entities and prodrugs that have novel effects, such as becoming active only when the pill reaches the gastrointestinal (GI) tract.
-
Combinations of these technologies.
The development of ADFs is an evolving area of research, and introduction and regulatory consideration of additional methods are expected.
ORDER A PLAGIARISM-FREE PAPER NOW
An industry-sponsored review by Michna and colleagues (2014) found that, relative to placebo, ADFs and non-ADFs were comparably effective and safe for individual patients with noncancer pain. However, it is important to understand that none of the available formulations is designed to prevent all types of misuse—for example, excessive oral ingestion is not prevented by an ADF designed to limit intravenous misuse. Interestingly, currently marketed ADF products do not claim on their labels that they are abuse-deterrent; rather, information on the label describes the studies that suggest abuse deterrence to inform prescribers. The reason is that there is no long-term evidence on the products’ real-world impact on reducing misuse, which the FDA would require for such a claim. Indeed, an FDA advisory committee recently voted to remove a particular formulation of oxycodone hydrochloride from the market, citing unexpectedly high potential for intravenous misuse (and associated public health harms) despite attempts to render the drug resistant to insufflation (FDA, 2017a). Thus, while ADFs represent a potentially promising area of opioid drug development, it remains aspirational.
For this reason, the FDA requires that manufacturers of all currently approved ADF products gather data demonstrating the magnitude of the products’ effect on real-world misuse relative to existing comparator products and the broader opioid ecosystem (FDA, 2015a). Multiple factors will determine the impact of any given ADF on public health through reduced prescription opioid misuse, addiction, and subsequent misuse of black market opioids. These include prescribing uptake and resulting market share, whether substitutions are made for other comparably harmful prescribed or illicit opioids, and whether ADFs are delivered to those patients with the highest risks of misuse. ADFs may do little to prevent misuse by determined individuals (or actions by a minority of dishonest prescribers), but may play an important role in preventing escalation to misuse.Managing the Risk of Opioid Prescription Essay. If evidence showed that abuse-deterrent opioids presented truly effective barriers to misuse and that patients with high risk of misuse or diversion were identifiable, one can envision clinical guidelines recommending the prescription of these formulations for such high-risk patients. It remains to be seen whether the FDA’s post-market research requirements for opioid manufacturers (see Annex 6-1 in Chapter 6), along with the ADF-specific data gathering mentioned previously, will eventually serve this purpose and reduce the misuse liability of individuals being prescribed opioids.
Another important question is whether the existence of relatively cheap heroin or fentanyl should be taken into account in deciding whether to phase out non-abuse-deterrent opioids, as has been strongly advocated by many analysts. While Severtson and colleagues (2013) report reductions in OxyContin-associated misuse and diversion following introduction of an ADF reformulation, Cicero and colleagues (2012) observe that indicators of fentanyl, hydromorphone, and heroin use went up during roughly the same period. Coplan and colleagues (2013) raise similar concerns based on National Poison System data, as do Cassidy and colleagues (2014) using data on 232,874 individuals assessed for substance use disorder treatment in 2008–2011. Coplan and colleagues (2016) examined the harms associated with reformulated OxyContin compared with other comparator prescription opioids, reporting a noticeable relative decrease for OxyContin, although this study did not specifically examine collateral outcomes such as potential transition to heroin and related harms. A recent state-by-state analysis suggests that the introduction of ADF OxyContin in 2010 resulted in reduced OxyContin misuse, but with a trade-off of increased heroin-related deaths and evidence of an overall trend of increased opioid overdose deaths (Alpert et al., 2017). Managing the Risk of Opioid Prescription Essay.
